2022年
No.4
PubMed (tuberculosis[Title/Abstract]) OR (lung cancer[Title/Abstract])
Filters applied: from 2022/4/1 - 2022/4/30.
1. Lancet. 2022 Apr 23;399(10335):1607-1617. doi: 10.1016/S0140-6736(21)02333-3.
Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung
cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised,
controlled, non-inferiority trial.
Saji H(1), Okada M(2), Tsuboi M(3), Nakajima R(4), Suzuki K(5), Aokage K(3),
Aoki T(6), Okami J(7), Yoshino I(8), Ito H(9), Okumura N(10), Yamaguchi M(11),
Ikeda N(12), Wakabayashi M(13), Nakamura K(13), Fukuda H(13), Nakamura S(14),
Mitsudomi T(15), Watanabe SI(16), Asamura H(17); West Japan Oncology Group and
Japan Clinical Oncology Group.
Author information:
(1)Department of Chest Surgery, St Marianna University School of Medicine,
Kawasaki, Japan. Electronic address: hsaji@marianna-u.ac.jp.
(2)Department of Surgical Oncology, Research Institute for Radiation Biology and
Medicine, Hiroshima University, Hiroshima, Japan.
(3)Department of Thoracic Surgery, National Cancer Center Hospital East,
Kashiwa, Japan.
…
BACKGROUND: Lobectomy is the standard of care for early-stage non-small-cell
lung cancer (NSCLC). The survival and clinical benefits of segmentectomy have
not been investigated in a randomised trial setting. We aimed to investigate if
segmentectomy was non-inferior to lobectomy in patients with small-sized
peripheral NSCLC.
METHODS: We conducted this randomised, controlled, non-inferiority trial at 70
institutions in Japan. Patients with clinical stage IA NSCLC (tumour diameter ≤2
cm; consolidation-to-tumour ratio >0·5) were randomly assigned 1:1 to receive
either lobectomy or segmentectomy. Randomisation was done via the minimisation
method, with balancing for the institution, histological type, sex, age, and
thin-section CT findings. Treatment allocation was not concealed from
investigators and patients. The primary endpoint was overall survival for all
randomly assigned patients. The secondary endpoints were postoperative
respiratory function (6 months and 12 months), relapse-free survival, proportion
of local relapse, adverse events, proportion of segmentectomy completion,
duration of hospital stay, duration of chest tube placement, duration of
surgery, amount of blood loss, and the number of automatic surgical staples
used. Overall survival was analysed on an intention-to-treat basis with a
non-inferiority margin of 1·54 for the upper limit of the 95% CI of the hazard
ratio (HR) and estimated using a stratified Cox regression model. This study is
registered with UMIN Clinical Trials Registry, UMIN000002317.
FINDINGS: Between Aug, 10, 2009, and Oct 21, 2014, 1106 patients
(intention-to-treat population) were enrolled to receive lobectomy (n=554) or
segmentectomy (n=552). Patient baseline clinicopathological factors were well
balanced between the groups. In the segmentectomy group, 22 patients were
switched to lobectomies and one patient received wide wedge resection. At a
median follow-up of 7·3 years (range 0·0-10·9), the 5-year overall survival was 94·3% (92·1-96·0) for segmentectomy and 91·1% for lobectomy (95% CI 88·4-93·2); superiority and non-inferiority in overall survival were confirmed using a stratified Cox regression model (HR 0·663; 95% CI 0·474-0·927; one-sided
p<0·0001 for non-inferiority; p=0·0082 for superiority). Improved overall
survival was observed consistently across all predefined subgroups in the
segmentectomy group. At 1 year follow-up, the significant difference in the
reduction of median forced expiratory volume in 1 sec between the two groups was
3·5% (p<0·0001), which did not reach the predefined threshold for clinical
significance of 10%. The 5-year relapse-free survival was 88·0% (95% CI
85·0-90·4) for segmentectomy and 87·9% (84·8-90·3) for lobectomy (HR 0·998; 95% CI 0·753-1·323; p=0·9889). The proportions of patients with local relapse were 10·5% for segmentectomy and 5·4% for lobectomy (p=0·0018). 52 (63%) of 83
patients and 27 (47%) of 58 patients died of other diseases after lobectomy and
segmentectomy, respectively. No 30-day or 90-day mortality was observed. One or
more postoperative complications of grade 2 or worse occurred at similar
frequencies in both groups (142 [26%] patients who received lobectomy, 148 [27%]
who received segmentectomy).
INTERPRETATION: To our knowledge, this study was the first phase 3 trial to show
the benefits of segmentectomy versus lobectomy in overall survival of patients
with small-peripheral NSCLC. The findings suggest that segmentectomy should be
the standard surgical procedure for this population of patients.
FUNDING: National Cancer Center Research and the Ministry of Health, Labour, and
Welfare of Japan.
Copyright © 2022 Elsevier Ltd. All rights reserved.
DOI: 10.1016/S0140-6736(21)02333-3
PMID: 35461558 [Indexed for MEDLINE]
2. N Engl J Med. 2022 May 26;386(21):1973-1985. doi: 10.1056/NEJMoa2202170. Epub
2022 Apr 11.
Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer.
Forde PM(1), Spicer J(1), Lu S(1), Provencio M(1), Mitsudomi T(1), Awad MM(1),
Felip E(1), Broderick SR(1), Brahmer JR(1), Swanson SJ(1), Kerr K(1), Wang C(1),
Ciuleanu TE(1), Saylors GB(1), Tanaka F(1), Ito H(1), Chen KN(1), Liberman M(1),
Vokes EE(1), Taube JM(1), Dorange C(1), Cai J(1), Fiore J(1), Jarkowski A(1),
Balli D(1), Sausen M(1), Pandya D(1), Calvet CY(1), Girard N(1); CheckMate 816
Investigators.
Author information:
(1)From the Bloomberg-Kimmel Institute for Cancer Immunotherapy, Johns Hopkins
Kimmel Cancer Center, Baltimore (P.M.F., S.R.B., J.R.B., J.M.T.); McGill
University Health Center (J.S.), and Centre Hospitalier de l'Université de
Montréal (M.L.) - both in Montreal; Shanghai Chest Hospital, School of Medicine,
Shanghai Jiao Tong University, Shanghai (S.L.), Tianjin Lung Cancer Center,
Tianjin Medical University Cancer Institute and Hospital, Tianjin (C.W.), and
Peking University School of Oncology, Beijing Cancer Hospital, Beijing (K.-N.C.)
- all in China; Hospital Universitario Puerta de Hierro, Madrid (M.P.); …
Comment in
N Engl J Med. 2022 May 26;386(21):2050-2051.
BACKGROUND: Neoadjuvant or adjuvant chemotherapy confers a modest benefit over
surgery alone for resectable non-small-cell lung cancer (NSCLC). In early-phase
trials, nivolumab-based neoadjuvant regimens have shown promising clinical
activity; however, data from phase 3 trials are needed to confirm these
findings.
METHODS: In this open-label, phase 3 trial, we randomly assigned patients with
stage IB to IIIA resectable NSCLC to receive nivolumab plus platinum-based
chemotherapy or platinum-based chemotherapy alone, followed by resection. The
primary end points were event-free survival and pathological complete response
(0% viable tumor in resected lung and lymph nodes), both evaluated by blinded
independent review. Overall survival was a key secondary end point. Safety was
assessed in all treated patients.
RESULTS: The median event-free survival was 31.6 months (95% confidence interval
[CI], 30.2 to not reached) with nivolumab plus chemotherapy and 20.8 months (95%
CI, 14.0 to 26.7) with chemotherapy alone (hazard ratio for disease progression,
disease recurrence, or death, 0.63; 97.38% CI, 0.43 to 0.91; P = 0.005). The
percentage of patients with a pathological complete response was 24.0% (95% CI,
18.0 to 31.0) and 2.2% (95% CI, 0.6 to 5.6), respectively (odds ratio, 13.94;
99% CI, 3.49 to 55.75; P<0.001). Results for event-free survival and
pathological complete response across most subgroups favored nivolumab plus
chemotherapy over chemotherapy alone. At the first prespecified interim
analysis, the hazard ratio for death was 0.57 (99.67% CI, 0.30 to 1.07) and did
not meet the criterion for significance. Of the patients who underwent
randomization, 83.2% of those in the nivolumab-plus-chemotherapy group and 75.4%
of those in the chemotherapy-alone group underwent surgery. Grade 3 or 4
treatment-related adverse events occurred in 33.5% of the patients in the
nivolumab-plus-chemotherapy group and in 36.9% of those in the
chemotherapy-alone group.
CONCLUSIONS: In patients with resectable NSCLC, neoadjuvant nivolumab plus
chemotherapy resulted in significantly longer event-free survival and a higher
percentage of patients with a pathological complete response than chemotherapy
alone. The addition of nivolumab to neoadjuvant chemotherapy did not increase
the incidence of adverse events or impede the feasibility of surgery. (Funded by
Bristol Myers Squibb; CheckMate 816 ClinicalTrials.gov number, NCT02998528.).
Copyright © 2022 Massachusetts Medical Society.
DOI: 10.1056/NEJMoa2202170
PMID: 35403841 [Indexed for MEDLINE]
3. Annu Rev Immunol. 2022 Apr 26;40:589-614. doi:
10.1146/annurev-immunol-093019-125148. Epub 2022 Feb 7.
The Tuberculous Granuloma and Preexisting Immunity.
Cohen SB(1), Gern BH(1)(2), Urdahl KB(1)(2)(3).
Author information:
(1)Seattle Children's Research Institute, Seattle, Washington, USA; email:
kevin.urdahl@seattlechildrens.org.
(2)Department of Pediatrics, University of Washington, Seattle, Washington, USA.
(3)Department of Immunology, University of Washington, Seattle, Washington, USA.
Pulmonary granulomas are widely considered the epicenters of the immune response
to Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB).
Recent animal studies have revealed factors that either promote or restrict TB
immunity within granulomas. These models, however, typically ignore the impact
of preexisting immunity on cellular organization and function, an important
consideration because most TB probably occurs through reinfection of previously
exposed individuals. Human postmortem research from the pre-antibiotic era
showed that infections in Mtb-naïve individuals (primary TB) versus those with
prior Mtb exposure (postprimary TB) have distinct pathologic features. We review
recent animal findings in TB granuloma biology, which largely reflect primary
TB. We also discuss our current understanding of postprimary TB lesions, about
which much less is known. Many knowledge gaps remain, particularly regarding how
preexisting immunity shapes granuloma structure and local immune responses at
Mtb infection sites.
DOI: 10.1146/annurev-immunol-093019-125148
PMID: 35130029 [Indexed for MEDLINE]
4. Nat Rev Microbiol. 2022 Apr 27:1-17. doi: 10.1038/s41579-022-00731-y. Online
ahead of print.
Anti-tuberculosis treatment strategies and drug development: challenges and
priorities.
Dartois VA(1), Rubin EJ(2).
Author information:
(1)Center for Discovery and Innovation, and Hackensack Meridian School of
Medicine, Department of Medical Sciences, Hackensack Meridian Health, Nutley,
NJ, USA. veronique.dartois@hmh-cdi.org.
(2)Harvard T.H. Chan School of Public Health, Department of Immunology and
Infectious Diseases, Boston, MA, USA.
Despite two decades of intensified research to understand and cure tuberculosis
disease, biological uncertainties remain and hamper progress. However, owing to
collaborative initiatives including academia, the pharmaceutical industry and
non-for-profit organizations, the drug candidate pipeline is promising. This
exceptional success comes with the inherent challenge of prioritizing multidrug
regimens for clinical trials and revamping trial designs to accelerate regimen
development and capitalize on drug discovery breakthroughs. Most wanted are
markers of progression from latent infection to active pulmonary disease,
markers of drug response and predictors of relapse, in vitro tools to uncover
synergies that translate clinically and animal models to reliably assess the
treatment shortening potential of new regimens. In this Review, we highlight the
benefits and challenges of 'one-size-fits-all' regimens and treatment duration
versus individualized therapy based on disease severity and host and pathogen
characteristics, considering scientific and operational perspectives.
© 2022. Springer Nature Limited.
DOI: 10.1038/s41579-022-00731-y
PMCID: PMC9045034
PMID: 35478222
5. Cancer Cell. 2022 May 9;40(5):479-493.e6. doi: 10.1016/j.ccell.2022.03.012. Epub 2022 Apr 21.
A phenotypic signature that identifies neoantigen-reactive T cells in fresh
human lung cancers.
Hanada KI(1), Zhao C(2), Gil-Hoyos R(2), Gartner JJ(2), Chow-Parmer C(2), Lowery
FJ(2), Krishna S(2), Prickett TD(2), Kivitz S(2), Parkhurst MR(2), Wong N(3),
Rae Z(4), Kelly MC(4), Goff SL(2), Robbins PF(2), Rosenberg SA(2), Yang JC(5).
Author information:
(1)Surgery Branch, Center for Cancer Research, National Cancer Institute,
National Institutes of Health, Bethesda, MD 20892, USA. Electronic address:
hanada@nih.gov.
(2)Surgery Branch, Center for Cancer Research, National Cancer Institute,
National Institutes of Health, Bethesda, MD 20892, USA.
(3)CCR Collaborative Bioinformatics Resource, Center for Cancer Research,
National Cancer Institute, National Institutes of Health, Bethesda, MD 20892,
USA; Advanced Biomedical Computational Science, Frederick National Laboratory
for Cancer Research, Leidos Biomedical Research, Frederick, MD 21701, USA.
…
A common theme across multiple successful immunotherapies for cancer is the
recognition of tumor-specific mutations (neoantigens) by T cells. The rapid
discovery of such antigen responses could lead to improved therapies through the
adoptive transfer of T cells engineered to express neoantigen-reactive T cell
receptors (TCRs). Here, through CITE-seq (cellular indexing of transcriptomes
and epitopes by sequencing) and TCR-seq of non-small cell lung cancer (NSCLC)
tumor-infiltrating lymphocytes (TILs), we develop a neoantigen-reactive T cell
signature based on clonotype frequency and CD39 protein and CXCL13 mRNA
expression. Screening of TCRs selected by the signature allows us to identify
neoantigen-reactive TCRs with a success rate of 45% for CD8+ and 66% for CD4+
T cells. Because of the small number of samples analyzed (4 patients),
generalizability remains to be tested. However, this approach can enable the
quick identification of neoantigen-reactive TCRs and expedite the engineering of
personalized neoantigen-reactive T cells for therapy.
Published by Elsevier Inc.
DOI: 10.1016/j.ccell.2022.03.012
PMCID: PMC9196205
PMID: 35452604 [Indexed for MEDLINE]
6. Nat Med. 2022 May;28(5):939-945. doi: 10.1038/s41591-022-01754-x. Epub 2022 Apr 14.
Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small
cell lung cancer: the phase 2 B-F1RST trial.
Kim ES(#)(1), Velcheti V(#)(2)(3), Mekhail T(4), Yun C(5), Shagan SM(5), Hu
S(5), Chae YK(6), Leal TA(7), Dowell JE(8), Tsai ML(9), Dakhil CSR(10), Stella
P(11), Jin Y(12), Shames DS(5), Schleifman E(5), Fabrizio DA(13), Phan S(5),
Socinski MA(4).
Author information:
(1)City of Hope National Medical Center, Los Angeles, CA, USA.
(2)Cleveland Clinic, Cleveland, OH, USA. vamsidhar.velcheti@nyulangone.org.
(3)New York University School of Medicine, New York, NY, USA.
vamsidhar.velcheti@nyulangone.org.
…
Tumor mutational burden (TMB) in circulating tumor DNA (ctDNA) has shown promise
in predicting benefit from PD-L1/PD-1 inhibitors in retrospective studies.
Aiming to assess blood TMB (bTMB) prospectively, we conducted B-F1RST (
NCT02848651 ), an open-label, phase 2 trial that evaluated bTMB as a predictive
biomarker for first-line atezolizumab monotherapy in locally advanced or
metastatic stage IIIB-IVB non-small cell lung cancer (n = 152). The co-primary
endpoints were investigator-assessed objective response rate (ORR) per RECIST
version 1.1 and investigator-assessed progression-free survival (PFS) between
high and low bTMB subgroups at the pre-defined bTMB ≥ 16 (14.5 mutations per
megabase) cutoff. Secondary endpoints included investigator-assessed PFS,
overall survival (OS) and duration of response at various bTMB cutoffs, as well
as safety. Investigator-assessed PFS in the bTMB ≥ 16 versus bTMB < 16 groups
was not statistically significant. However, bTMB ≥ 16 was associated with higher
ORR, and ORR improved as bTMB cutoffs increased. No new safety signals were
seen. In exploratory analyses, patients with maximum somatic allele frequency
(MSAF) < 1% had higher ORR than patients with MSAF ≥ 1%. However, further
analysis showed that this effect was driven by better baseline prognostics
rather than by MSAF itself. At 36.5-month follow-up, an exploratory analysis of
OS found that bTMB ≥ 16 was associated with longer OS than bTMB < 16. Further
study and assay optimization will be required to develop bTMB as a predictive,
standalone biomarker of immunotherapy or for use in conjunction with other
biomarkers.
© 2022. The Author(s).
DOI: 10.1038/s41591-022-01754-x
PMCID: PMC9117143
PMID: 35422531 [Indexed for MEDLINE]
7. Nat Rev Microbiol. 2022 Apr 1. doi: 10.1038/s41579-022-00721-0. Online ahead of print.
Types and functions of heterogeneity in mycobacteria.
Chung ES(1), Johnson WC(1)(2), Aldridge BB(3)(4)(5)(6).
Author information:
(1)Department of Molecular Biology and Microbiology, Tufts University School of
Medicine, Boston, MA, USA.
(2)Tufts University School of Graduate Biomedical Sciences, Boston, MA, USA.
(3)Department of Molecular Biology and Microbiology, Tufts University School of
Medicine, Boston, MA, USA. bree.aldridge@tufts.edu.
…
The remarkable ability of Mycobacterium tuberculosis to survive attacks from the
host immune response and drug treatment is due to the resilience of a few
bacilli rather than a result of survival of the entire population. Maintenance
of mycobacterial subpopulations with distinct phenotypic characteristics is key
for survival in the face of dynamic and variable stressors encountered during
infection. Mycobacterial populations develop a wide range of phenotypes through
an innate asymmetric growth pattern and adaptation to fluctuating
microenvironments during infection that point to heterogeneity being a vital
survival strategy. In this Review, we describe different types of mycobacterial
heterogeneity and discuss how heterogeneity is generated and regulated in
response to environmental cues. We discuss how this heterogeneity may have a key
role in recording memory of their environment at both the single-cell level and
the population level to give mycobacterial populations plasticity to withstand
complex stressors.
© 2022. Springer Nature Limited.
DOI: 10.1038/s41579-022-00721-0
PMID: 35365812
8. Immunity. 2022 May 10;55(5):827-846.e10. doi: 10.1016/j.immuni.2022.04.004. Epub 2022 Apr 27.
Multimodal profiling of lung granulomas in macaques reveals cellular correlates
of tuberculosis control.
Gideon HP(1), Hughes TK(2), Tzouanas CN(2), Wadsworth MH 2nd(3), Tu AA(4),
Gierahn TM(4), Peters JM(5), Hopkins FF(6), Wei JR(6), Kummerlowe C(7), Grant
NL(8), Nargan K(9), Phuah JY(8), Borish HJ(8), Maiello P(8), White AG(8),
Winchell CG(10), Nyquist SK(11), Ganchua SKC(8), Myers A(8), Patel KV(8), Ameel
CL(8), Cochran CT(8), Ibrahim S(2), Tomko JA(8), Frye LJ(8), Rosenberg JM(12),
Shih A(13), Chao M(6), Klein E(14), Scanga CA(1), Ordovas-Montanes J(15), Berger
B(16), Mattila JT(17), Madansein R(18), Love JC(19), Lin PL(20), Leslie A(21),
Behar SM(22), Bryson B(5), Flynn JL(23), Fortune SM(24), Shalek AK(25).
Author information:
(1)Department of Microbiology and Molecular Genetics, University of Pittsburgh
School of Medicine, Pittsburgh, PA, USA; Center for Vaccine Research, University
of Pittsburgh, Pittsburgh, PA, USA.
(2)Institute for Medical Engineering & Science, Massachusetts Institute of
Technology, Cambridge, MA, USA; Ragon Institute of MGH, MIT, and Harvard,
Cambridge, MA, USA; Broad Institute of MIT and Harvard, Cambridge, MA, USA.
(3)Institute for Medical Engineering & Science, Massachusetts Institute of
Technology, Cambridge, MA, USA; Ragon Institute of MGH, MIT, and Harvard,
Cambridge, MA, USA; Broad Institute of MIT and Harvard, Cambridge, MA, USA;
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA,
USA.
…
Comment in
Immunity. 2022 May 10;55(5):819-821.
Mycobacterium tuberculosis lung infection results in a complex multicellular
structure: the granuloma. In some granulomas, immune activity promotes bacterial
clearance, but in others, bacteria persist and grow. We identified correlates of
bacterial control in cynomolgus macaque lung granulomas by co-registering
longitudinal positron emission tomography and computed tomography imaging,
single-cell RNA sequencing, and measures of bacterial clearance. Bacterial
persistence occurred in granulomas enriched for mast, endothelial, fibroblast,
and plasma cells, signaling amongst themselves via type 2 immunity and
wound-healing pathways. Granulomas that drove bacterial control were
characterized by cellular ecosystems enriched for type 1-type 17, stem-like, and
cytotoxic T cells engaged in pro-inflammatory signaling networks involving
diverse cell populations. Granulomas that arose later in infection displayed
functional characteristics of restrictive granulomas and were more capable of
killing Mtb. Our results define the complex multicellular ecosystems underlying
(lack of) granuloma resolution and highlight host immune targets that can be
leveraged to develop new vaccine and therapeutic strategies for TB.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
DOI: 10.1016/j.immuni.2022.04.004
PMCID: PMC9122264
PMID: 35483355 [Indexed for MEDLINE]
9. Adv Mater. 2022 Apr 28:e2201516. doi: 10.1002/adma.202201516. Online ahead of
print.
Targeted Drug/Gene/Photodynamic Therapy via a Stimuli-Responsive
Dendritic-Polymer-Based Nanococktail for Treatment of EGFR-TKI-Resistant
Non-Small-Cell Lung Cancer.
Huang J(1), Zhuang C(1), Chen J(1), Chen X(1), Li X(1), Zhang T(1), Wang B(1),
Feng Q(1), Zheng X(1), Gong M(1)(2), Gong Q(1)(3), Xiao K(1)(3), Luo K(1)(3), Li
W(1)(3).
Author information:
(1)Precision Medicine Research Center, Huaxi MR Research Center (HMRRC),
Frontiers Science Center for Disease-Related Molecular Network, National
Clinical Research Center for Geriatrics, Department of Respiratory Medicine and
Department of Radiology, State Key Laboratory of Biotherapy, West China
Hospital, Sichuan University, Chengdu, 610041, China.
(2)West China-Washington Mitochondria and Metabolism Research Center, West China
Hospital, Sichuan University, Chengdu, 610041, China.
(3)Sichuan Provincial Key Laboratory of Precision Medicine, Functional and
Molecular Imaging Key Laboratory of Sichuan Province, and Research Unit of
Psychoradiology, Chinese Academy of Medical Sciences, Chengdu, 610041, China.
Yes-associated protein (YAP) has been identified as a key driver for epidermal
growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) resistance.
Inhibition of YAP expression could be a potential therapeutic option for
treating non-small-cell lung cancer (NSCLC). Herein, a nanococktail therapeutic
strategy is proposed by employing amphiphilic and block-dendritic-polymer-based
nanoparticles (NPs) for targeted co-delivery of EGFR-TKI gefitinib (Gef) and
YAP-siRNA to achieve a targeted drug/gene/photodynamic therapy. The resulting
NPs are effectively internalized into Gef-resistant NSCLC cells, successfully
escape from late endosomes/lysosomes, and responsively release Gef and YAP-siRNA
in an intracellular reductive environment. They preferentially accumulate at the
tumor site after intravenous injection in both cell-line-derived xenograft (CDX)
and patient-derived xenograft (PDX) models of Gef-resistant NSCLC, resulting in
potent antitumor efficacy without distinct toxicity after laser irradiation.
Mechanism studies reveal that the cocktail therapy could block the EGFR
signaling pathway with Gef, inhibit activation of the EGFR bypass signaling
pathway via YAP-siRNA, and induce tumor cell apoptosis through photodynamic
therapy (PDT). Furthermore, this combination nanomedicine can sensitize PDT and
impair glycolysis by downregulating HIF-1α. These results suggest that this
stimuli-responsive dendritic-polymer-based nanococktail therapy may provide a
promising approach for the treatment of EGFR-TKI resistant NSCLC.
© 2022 Wiley-VCH GmbH.
DOI: 10.1002/adma.202201516
PMID: 35481881
10. Nat Commun. 2022 Apr 26;13(1):2255. doi: 10.1038/s41467-022-29873-6.
A periplasmic cinched protein is required for siderophore secretion and
virulence of Mycobacterium tuberculosis.
Zhang L(1), Kent JE(2), Whitaker M(3), Young DC(4), Herrmann D(1), Aleshin
AE(2), Ko YH(5), Cingolani G(5), Saad JS(1), Moody DB(4), Marassi FM(2), Ehrt
S(3), Niederweis M(6).
Author information:
(1)Department of Microbiology, University of Alabama at Birmingham, Birmingham,
AL, 35294, USA.
(2)Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla,
CA, 92037, USA.
(3)Department of Microbiology and Immunology, Weill Cornell Medical College, New
York, NY, 10021, USA.
…
Iron is essential for growth of Mycobacterium tuberculosis, the causative agent
of tuberculosis. To acquire iron from the host, M. tuberculosis uses the
siderophores called mycobactins and carboxymycobactins. Here, we show that the
rv0455c gene is essential for M. tuberculosis to grow in low-iron medium and
that secretion of both mycobactins and carboxymycobactins is drastically reduced
in the rv0455c deletion mutant. Both water-soluble and membrane-anchored Rv0455c
are functional in siderophore secretion, supporting an intracellular role. Lack
of Rv0455c results in siderophore toxicity, a phenotype observed for other
siderophore secretion mutants, and severely impairs replication of M.
tuberculosis in mice, demonstrating the importance of Rv0455c and siderophore
secretion during disease. The crystal structure of a Rv0455c homolog reveals a
novel protein fold consisting of a helical bundle with a 'cinch' formed by an
essential intramolecular disulfide bond. These findings advance our
understanding of the distinct M. tuberculosis siderophore secretion system.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29873-6
PMCID: PMC9042941
PMID: 35474308 [Indexed for MEDLINE]
11. Nat Commun. 2022 Apr 22;13(1):2206. doi: 10.1038/s41467-022-29905-1.
A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1
inactive lung cancers.
Koppula P(#)(1)(2), Lei G(#)(1), Zhang Y(1), Yan Y(1), Mao C(1), Kondiparthi
L(3), Shi J(4), Liu X(1), Horbath A(1), Das M(1), Li W(4), Poyurovsky MV(3),
Olszewski K(3)(5), Gan B(6)(7).
Author information:
(1)Department of Experimental Radiation Oncology, The University of Texas MD
Anderson Cancer Center, Houston, TX, 77030, USA.
(2)The University of Texas MD Anderson UTHealth Graduate School of Biomedical
Sciences, Houston, TX, 77030, USA.
(3)Kadmon Corporation, LLC, New York, NY, 10016, USA.
…
Targeting ferroptosis, a unique cell death modality triggered by unrestricted
lipid peroxidation, in cancer therapy is hindered by our incomplete
understanding of ferroptosis mechanisms under specific cancer genetic contexts.
KEAP1 (kelch-like ECH associated protein 1) is frequently mutated or inactivated
in lung cancers, and KEAP1 mutant lung cancers are refractory to most therapies,
including radiotherapy. In this study, we identify ferroptosis suppressor
protein 1 (FSP1, also known as AIFM2) as a transcriptional target of nuclear
factor erythroid 2-related factor 2 (NRF2) and reveal that the ubiquinone
(CoQ)-FSP1 axis mediates ferroptosis- and radiation- resistance in KEAP1
deficient lung cancer cells. We further show that pharmacological inhibition of
the CoQ-FSP1 axis sensitizes KEAP1 deficient lung cancer cells or
patient-derived xenograft tumors to radiation through inducing ferroptosis.
Together, our study identifies CoQ-FSP1 as a key downstream effector of
KEAP1-NRF2 pathway and as a potential therapeutic target for treating KEAP1
mutant lung cancers.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29905-1
PMCID: PMC9033817
PMID: 35459868 [Indexed for MEDLINE]
12. Nat Commun. 2022 Apr 22;13(1):2203. doi: 10.1038/s41467-022-29832-1.
CinA mediates multidrug tolerance in Mycobacterium tuberculosis.
Kreutzfeldt KM(1), Jansen RS(2)(3), Hartman TE(2), Gouzy A(1), Wang R(1)(4),
Krieger IV(5), Zimmerman MD(6), Gengenbacher M(6), Sarathy JP(6), Xie M(6),
Dartois V(6), Sacchettini JC(5), Rhee KY(2)(1), Schnappinger D(7), Ehrt S(8).
Author information:
(1)Department of Microbiology and Immunology, Weill Cornell Medical College, New
York, NY, 10065, USA.
(2)Division of Infectious Diseases, Department of Medicine, Weill Cornell
Medical College, New York, NY, 10065, USA.
(3)Department of Microbiology, Radboud University, 6525 AJ, Nijmegen, The
Netherlands.
…
The ability of Mycobacterium tuberculosis (Mtb) to resist and tolerate
antibiotics complicates the development of improved tuberculosis (TB)
chemotherapies. Here we define the Mtb protein CinA as a major determinant of
drug tolerance and as a potential target to shorten TB chemotherapy. By reducing
the fraction of drug-tolerant persisters, genetic inactivation of cinA
accelerated killing of Mtb by four antibiotics in clinical use: isoniazid,
ethionamide, delamanid and pretomanid. Mtb ΔcinA was killed rapidly in
conditions known to impede the efficacy of isoniazid, such as during nutrient
starvation, during persistence in a caseum mimetic, in activated macrophages and
during chronic mouse infection. Deletion of CinA also increased in vivo killing
of Mtb by BPaL, a combination of pretomanid, bedaquiline and linezolid that is
used to treat highly drug-resistant TB. Genetic and drug metabolism studies
suggest that CinA mediates drug tolerance via cleavage of NAD-drug adducts.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29832-1
PMCID: PMC9033802
PMID: 35459278 [Indexed for MEDLINE]
13. J Clin Oncol. 2022 Apr 22:JCO2200227. doi: 10.1200/JCO.22.00227. Online ahead of print.
COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or
in Combination With Oleclumab or Monalizumab in Patients With Unresectable,
Stage III Non-Small-Cell Lung Cancer.
Herbst RS(1), Majem M(2), Barlesi F(3), Carcereny E(4), Chu Q(5), Monnet I(6),
Sanchez-Hernandez A(7), Dakhil S(8), Camidge DR(9), Winzer L(10), Soo-Hoo Y(11),
Cooper ZA(11), Kumar R(11), Bothos J(11), Aggarwal C(12), Martinez-Marti A(13).
Author information:
(1)Yale Cancer Center, New Haven, CT.
(2)Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
(3)Gustave Roussy, Villejuif, France.
…
PURPOSE: Durvalumab significantly improves overall survival for patients with
unresectable stage III non-small-cell lung cancer and no progression after
concurrent chemoradiotherapy (cCRT). Building upon that standard of care, COAST
is a phase II study of durvalumab alone or combined with the anti-CD73
monoclonal antibody oleclumab or anti-NKG2A monoclonal antibody monalizumab as
consolidation therapy in this setting.
METHODS: Patients with unresectable stage III non-small-cell lung cancer,
Eastern Cooperative Oncology Group performance status 0/1, and no progression
after cCRT were randomly assigned 1:1:1, ≤ 42 days post-cCRT, to durvalumab
alone or combined with oleclumab or monalizumab for up to 12 months, stratified
by histology. The primary end point was investigator-assessed confirmed
objective response rate (ORR; RECIST v1.1).
RESULTS: Between January 2019 and July 2020, 189 patients were randomly
assigned. At this interim analysis (data cutoff, May 17, 2021), median follow-up
was 11.5 months (range, 0.4-23.4 months; all patients). Confirmed ORR was
numerically higher with durvalumab plus oleclumab (30.0%; 95% CI, 18.8 to 43.2)
and durvalumab plus monalizumab (35.5%; 95% CI, 23.7 to 48.7) versus durvalumab
(17.9%; 95% CI, 9.6 to 29.2). Progression-free survival (PFS) was prolonged with
both combinations versus durvalumab (plus oleclumab: hazard ratio, 0.44; 95% CI,
0.26 to 0.75; and plus monalizumab: hazard ratio, 0.42; 95% CI, 0.24 to 0.72),
with higher 12-month PFS rates (plus oleclumab: 62.6% [95% CI, 48.1 to 74.2] and
plus monalizumab: 72.7% [95% CI, 58.8 to 82.6] v durvalumab alone: 33.9% [95%
CI, 21.2 to 47.1]). All-cause grade ≥ 3 treatment-emergent adverse events
occurred in 40.7%, 27.9%, and 39.4% with durvalumab plus oleclumab, durvalumab
plus monalizumab, and durvalumab, respectively.
CONCLUSION: Both combinations increased ORR and prolonged PFS versus durvalumab
alone. Safety was similar across arms with no new or significant safety signals
identified with either combination. These data support their further evaluation
in a phase III trial.
DOI: 10.1200/JCO.22.00227
PMID: 35452273
14. Nat Commun. 2022 Apr 20;13(1):2154. doi: 10.1038/s41467-022-29647-0.
Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated
CAR-T cell migration.
Li H(1)(2), Harrison EB(1)(3), Li H(1)(4), Hirabayashi K(1), Chen J(5), Li
QX(5), Gunn J(5), Weiss J(1)(6)(7), Savoldo B(1)(8), Parker JS(1)(9), Pecot
CV(1)(6)(7), Dotti G(10)(11), Du H(12)(13).
Author information:
(1)Lineberger Comprehensive Cancer Center, University of North Carolina at
Chapel Hill, Chapel Hill, NC, USA.
(2)Department of Medical Oncology, Beijing Chest Hospital, Capital Medical
University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing,
China.
(3)Center for Nanotechnology in Drug Delivery, Eshelman School of Pharmacy,
University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
…
Metastatic non-small cell lung cancer (NSCLC) remains largely incurable and the
prognosis is extremely poor once it spreads to the brain. In particular, in
patients with brain metastases, the blood brain barrier (BBB) remains a
significant obstacle for the biodistribution of antitumor drugs and immune
cells. Here we report that chimeric antigen receptor (CAR) T cells targeting
B7-H3 (B7-H3.CAR) exhibit antitumor activity in vitro against tumor cell lines
and lung cancer organoids, and in vivo in xenotransplant models of orthotopic
and metastatic NSCLC. The co-expression of the CCL2 receptor CCR2b in
B7-H3.CAR-T cells, significantly improves their capability of passing the BBB,
providing enhanced antitumor activity against brain tumor lesions. These
findings indicate that leveraging T-cell chemotaxis through CCR2b co-expression
represents a strategy to improve the efficacy of adoptive T-cell therapies in
patients with solid tumors presenting with brain metastases.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29647-0
PMCID: PMC9021299
PMID: 35443752 [Indexed for MEDLINE]
15. Nat Commun. 2022 Apr 19;13(1):2023. doi: 10.1038/s41467-022-29517-9.
Heterogeneity of neuroendocrine transcriptional states in metastatic small cell
lung cancers and patient-derived models.
Lissa D(#)(1), Takahashi N(#)(2)(3), Desai P(2), Manukyan I(4), Schultz CW(2),
Rajapakse V(2), Velez MJ(5), Mulford D(6), Roper N(2), Nichols S(2), Vilimas
R(2), Sciuto L(2), Chen Y(7), Guha U(8), Rajan A(8), Atkinson D(9), El Meskini
R(9), Weaver Ohler Z(9), Thomas A(10).
Author information:
(1)Laboratory of Human Carcinogenesis, Center for Cancer Research, NCI,
Bethesda, MD, 20892, USA.
(2)Developmental Therapeutics Branch, Center for Cancer Research, NCI, Bethesda,
MD, 20892, USA.
(3)Medical Oncology Department, Center Hospital, National Center for Global
Health and Medicine, Tokyo, Japan.
…
Molecular subtypes of small cell lung cancer (SCLC) defined by the expression of
key transcription regulators have recently been proposed in cell lines and
limited number of primary tumors. The clinical and biological implications of
neuroendocrine (NE) subtypes in metastatic SCLC, and the extent to which they
vary within and between patient tumors and in patient-derived models is not
known. We integrate histology, transcriptome, exome, and treatment outcomes of
SCLC from a range of metastatic sites, revealing complex intra- and intertumoral
heterogeneity of NE differentiation. Transcriptomic analysis confirms previously
described subtypes based on ASCL1, NEUROD1, POU2F3, YAP1, and ATOH1 expression,
and reveal a clinical subtype with hybrid NE and non-NE phenotypes, marked by
chemotherapy-resistance and exceedingly poor outcomes. NE tumors are more likely
to have RB1, NOTCH, and chromatin modifier gene mutations, upregulation of DNA
damage response genes, and are more likely to respond to replication stress
targeted therapies. In contrast, patients preferentially benefited from
immunotherapy if their tumors were non-NE. Transcriptional phenotypes strongly
skew towards the NE state in patient-derived model systems, an observation that
was confirmed in paired patient-matched tumors and xenografts. We provide a
framework that unifies transcriptomic and genomic dimensions of metastatic SCLC.
The marked differences in transcriptional diversity between patient tumors and
model systems are likely to have implications in development of novel
therapeutic agents.
© 2022. This is a U.S. Government work and not under copyright protection in the
US; foreign copyright protection may apply.
DOI: 10.1038/s41467-022-29517-9
PMCID: PMC9018864
PMID: 35440132 [Indexed for MEDLINE]
16. Nat Commun. 2022 Apr 19;13(1):2144. doi: 10.1038/s41467-022-29794-4.
Genomic and transcriptomic analysis of a library of small cell lung cancer
patient-derived xenografts.
Caeser R(1), Egger JV(1), Chavan S(1), Socci ND(2), Jones CB(2), Kombak FE(3),
Asher M(3), Roehrl MH(3), Shah NS(1), Allaj V(1), Manoj P(1), Tischfield SE(4),
Kulick A(5), Meneses M(5), Iacobuzio-Donahue CA(3)(6)(7), Lai WV(1), Bhanot
U(3), Baine MK(6), Rekhtman N(6), Hollmann TJ(6)(7), de Stanchina E(5), Poirier
JT(8), Rudin CM(9)(10), Sen T(11).
Author information:
(1)Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY,
10065, USA.
(2)Bioinformatics Core, Memorial Sloan Kettering Cancer Center, New York, NY,
10065, USA.
(3)Precision Pathology Center, Memorial Sloan Kettering Cancer Center, New York,
NY, 10065, USA.
…
Access to clinically relevant small cell lung cancer (SCLC) tissue is limited
because surgical resection is rare in metastatic SCLC. Patient-derived
xenografts (PDX) and circulating tumor cell-derived xenografts (CDX) have
emerged as valuable tools to characterize SCLC. Here, we present a resource of
46 extensively annotated PDX/CDX models derived from 33 patients with SCLC. We
perform multi-omic analyses, using targeted tumor next-generation sequencing,
RNA-sequencing, and immunohistochemistry to deconvolute the mutational
landscapes, global expression profiles, and molecular subtypes of these SCLC
models. SCLC subtypes characterized by transcriptional regulators, ASCL1,
NEUROD1 and POU2F3 are confirmed in this cohort. A subset of SCLC clinical
specimens, including matched PDX/CDX and clinical specimen pairs, confirm that
the primary features and genomic and proteomic landscapes of the tumors of
origin are preserved in the derivative PDX models. This resource provides a
powerful system to study SCLC biology.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29794-4
PMCID: PMC9018685
PMID: 35440124 [Indexed for MEDLINE]
17. J Am Chem Soc. 2022 Apr 27;144(16):7117-7128. doi: 10.1021/jacs.1c12075. Epub
2022 Apr 13.
Highly Potent, Selective, Biostable, and Cell-Permeable Cyclic d-Peptide for
Dual-Targeting Therapy of Lung Cancer.
Zhou Y(1), Zou Y(1), Yang M(1), Mei S(1), Liu X(1), Han H(1), Zhang CD(1), Niu
MM(1).
Author information:
(1)Key Laboratory of Drug Quality Control and Pharmacovigilance, Ministry of
Education, State Key Laboratory of Natural Medicines, School of Basic Medicine
and Clinical Pharmacy, China Pharmaceutical University, Nanjing 210009, China.
The application of peptide drugs in cancer therapy is impeded by their poor
biostability and weak cell permeability. Therefore, it is imperative to find
biostable and cell-permeable peptide drugs for cancer treatment. Here, we
identified a potent, selective, biostable, and cell-permeable cyclic d-peptide,
NKTP-3, that targets NRP1 and KRASG12D using structure-based virtual screening.
NKTP-3 exhibited strong biostability and cellular uptake ability. Importantly,
it significantly inhibited the growth of A427 cells with the KRASG12D mutation.
Moreover, NKTP-3 showed strong antitumor activity against A427 cell-derived
xenograft and KRASG12D-driven primary lung cancer models without obvious
toxicity. This study demonstrates that the dual NRP1/KRASG12D-targeting cyclic
d-peptide NKTP-3 may be used as a potential chemotherapeutic agent for
KRASG12D-driven lung cancer treatment.
DOI: 10.1021/jacs.1c12075
PMID: 35417174 [Indexed for MEDLINE]
18. Am J Respir Crit Care Med. 2022 Apr 12. doi: 10.1164/rccm.202112-2747OC. Online ahead of print.
Response to Hypoxia and the Ensuing Dysregulation of Inflammation Impacts
Mycobacterium tuberculosis Pathogenicity.
Bucşan AN(1), Veatch A(1), Singh DK(2), Akter S(3), Golden NA(4), Kirkpatrick
M(1), Threeton B(1), Moodley C(1), Ahmed M(3), Doyle LA(5), Russell-Lodrigue
K(5), Norton EB(1), Didier PJ(6), Roy CJ(7), Abramovitch RB(8), Mehra S(9),
Khader SA(10), Kaushal D(11).
Author information:
(1)Tulane National Primate Research Center, 101404, Covington, Louisiana, United
States.
(2)Texas Biomedical Research Institute, 7075, Southwest National Primate
Research Center, San Antonio, Texas, United States.
(3)Washington University School of Medicine in Saint Louis, 12275, St Louis,
Missouri, United States.
…
RATIONALE: Different Mycobacterium tuberculosis (Mtb) strains exhibit variable
levels of virulence in humans and animal models. Differing stress response
strategies employed by different strains of Mtb could influence virulence.
OBJECTIVES: We compared the virulence of two strains of Mtb with use in animal
models research: CDC1551 and Erdman.
METHODS: Rhesus macaques, which develop human-like TB attributes and pathology,
were infected with a high-dose of either strain via aerosol, and virulence
compared by bacterial burden and pathology.
MEASUREMENTS AND MAIN RESULTS: Infection with Erdman resulted in significantly
shorter times to euthanasia and higher bacterial burdens, greater systemic
inflammation, and lung pathology, relative to those infected with CDC1551.
Macaques infected with Erdman also exhibited significantly higher, early
inflammatory myeloid cell influx to the lung; greater macrophage and T cell
activity; and higher expression of lung remodeling (ECM) genes, consistent with
greater pathology. Expression of NOTCH4 signaling, which is induced in response
to hypoxia and promotes undifferentiated cellular state, was also higher in
Erdman-infected lungs. The granulomas generated by Erdman-, and not
CDC1551-infection appeared to have larger regions of necrosis, which is strongly
associated with hypoxia. To better understand the mechanisms of differential
hypoxia induction by these strains, we subjected both to hypoxia in-vitro.
Erdman induced higher levels of DosRST regulon relative to CDC1551. The DosRST
regulon is the global regulator of response to hypoxia in Mtb and critical for
its persistence in granulomas.
CONCLUSIONS: Our results show that the response to hypoxia is a critical
mediator of virulence determination in Mtb with potential impacts on bacillary
persistence, reactivation and efficiency of therapeutics. This article is open
access and distributed under the terms of the Creative Commons Attribution 4.0
International License (https://creativecommons.org/licenses/by/4.0/).
DOI: 10.1164/rccm.202112-2747OC
PMID: 35412961
19. Cancer Discov. 2022 Apr 11:candisc.1615.2021. doi: 10.1158/2159-8290.CD-21-1615. Online ahead of print.
Sunvozertinib, a selective EGFR inhibitor for previously treated non-small cell
lung cancer with EGFR exon 20 insertion mutations.
Wang M(1), Yang JC(2), Mitchell PL(3), Fang J(4), Camidge DR(5), Nian W(6), Chiu
CH(7), Zhou J(8), Zhao Y(9), Su WC(10), Yang TY(11), Zhu VW(12), Millward M(13),
Fan Y(14), Huang WT(15), Cheng Y(16), Jiang L(17), Brungs D(18), Bazhenova
L(19), Lee CK(20), Gao B(21), Xu Y(1), Hsu WH(22), Zheng L(23), Janne PA(24).
Author information:
(1)Peking Union Medical College Hospital, Chinese Academy of Medical Sciences
and Peking Union Medical College, Beijing, China.
(2)National Taiwan University Hospital and National Taiwan University Cancer
Center, Taipei, Taiwan, Taipei, Taiwan.
(3)Austin Health, Melbourne, VIC, Australia.
…
Epidermal growth factor receptor exon 20 insertion mutations (EGFR exon20ins)
are detected in approximately 2% of patients with non-small cell lung cancer
(NSCLC). Due to lack of effective therapy, the prognosis of these patients was
poor. Sunvozertinib (DZD9008) was designed as an oral, potent, irreversible and
selective EGFR tyrosine kinase inhibitor, showing activity against EGFR
exon20ins and other mutations. In both cell lines and xenograft models,
sunvozertinib shows potent antitumor activity. In the two ongoing phase 1
clinical studies, sunvozertinib was tolerated up to 400 mg once daily. The most
common drug-related adverse events included diarrhea and skin rash. Antitumor
efficacy was observed at the doses of 100 mg and above in patients with EGFR
exon20ins NSCLC across different subtypes, with prior amivantamab treatment as
well as with baseline brain metastasis. The median duration of response (DoR)
has not been reached.
DOI: 10.1158/2159-8290.CD-21-1615
PMID: 35404393
20. Sci Transl Med. 2022 Apr 6;14(639):eabj4124. doi: 10.1126/scitranslmed.abj4124. Epub 2022 Apr 6.
Point-of-care diagnostic tests for tuberculosis disease.
Hong JM(1), Lee H(1), Menon NV(2), Lim CT(2)(3)(4), Lee LP(5)(6)(7)(8)(9)(10),
Ong CWM(1)(3).
Author information:
(1)Infectious Diseases Translational Research Programme, Department of Medicine,
Yong Loo Lin School of Medicine, National University of Singapore, Singapore
117599, Singapore.
(2)Department of Biomedical Engineering, National University of Singapore,
Singapore 117583, Singapore.
(3)Institute for Health Innovation & Technology (iHealthtech), National
University of Singapore, Singapore 117599, Singapore.
…
Rapid diagnosis is one key pillar to end tuberculosis (TB). Point-of-care tests
(POCTs) facilitate early detection, immediate treatment, and reduced
transmission of TB disease. This Review evaluates current diagnostic assays
endorsed by the World Health Organization and identifies the gaps between
existing conventional tests and the ideal POCT. We discuss the commercial
development of new rapid tests and research studies on nonsputum-based
diagnostic biomarkers from both pathogen and host. Last, we highlight advances
in integrated microfluidics technology that may aid the development of new
POCTs.
DOI: 10.1126/scitranslmed.abj4124
PMID: 35385338 [Indexed for MEDLINE]
21. Nat Commun. 2022 Apr 5;13(1):1811. doi: 10.1038/s41467-022-29444-9.
Integrative analysis of non-small cell lung cancer patient-derived xenografts
identifies distinct proteotypes associated with patient outcomes.
Mirhadi S(1)(2), Tam S(3), Li Q(3), Moghal N(3), Pham NA(3), Tong J(1), Golbourn
BJ(4), Krieger JR(5), Taylor P(1), Li M(3), Weiss J(6), Martins-Filho SN(3)(7),
Raghavan V(3), Mamatjan Y(3), Khan AA(1), Cabanero M(3)(7), Sakashita S(3), Huo
K(3), Agnihotri S(4), Ishizawa K(8), Waddell TK(8)(9), Zadeh G(3)(9), Yasufuku
K(8)(9), Liu G(3)(10)(11), Shepherd FA(3)(10), Moran MF(12)(13)(14), Tsao
MS(15)(16)(17).
Author information:
(1)Program in Cell Biology, Hospital for Sick Children, Toronto, ON, Canada.
(2)Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada.
(3)Princess Margaret Cancer Centre, University Health Network, Toronto, ON,
Canada.
…
Non-small cell lung cancer (NSCLC) is the leading cause of cancer deaths
worldwide. Only a fraction of NSCLC harbor actionable driver mutations and there
is an urgent need for patient-derived model systems that will enable the
development of new targeted therapies. NSCLC and other cancers display profound
proteome remodeling compared to normal tissue that is not predicted by DNA or
RNA analyses. Here, we generate 137 NSCLC patient-derived xenografts (PDXs) that
recapitulate the histology and molecular features of primary NSCLC. Proteome
analysis of the PDX models reveals 3 adenocarcinoma and 2 squamous cell
carcinoma proteotypes that are associated with different patient outcomes,
protein-phosphotyrosine profiles, signatures of activated pathways and candidate
targets, and in adenocarcinoma, stromal immune features. These findings portend
proteome-based NSCLC classification and treatment and support the PDX resource
as a viable model for the development of new targeted therapies.
© 2022. The Author(s).
DOI: 10.1038/s41467-022-29444-9
PMCID: PMC8983714
PMID: 35383171 [Indexed for MEDLINE]
22. Lancet Infect Dis. 2022 Apr;22(4):e108-e120. doi: 10.1016/S1473-3099(21)00810-0. Epub 2022 Feb 28.
Accelerating research and development of new vaccines against tuberculosis: a
global roadmap.
Cobelens F(1), Suri RK(2), Helinski M(3), Makanga M(3), Weinberg AL(3),
Schaffmeister B(4), Deege F(4), Hatherill M(5); TB Vaccine Roadmap Stakeholder
Group.
Author information:
(1)Department of Global Health and Amsterdam Institute for Global Health and
Development, Amsterdam University Medical Centers, Amsterdam, Netherlands.
Electronic address: f.g.cobelens@amsterdamumc.nl.
(2)Department of Governance and Strategy, Developing Countries Vaccine
Manufacturers' Network International, Nyon, Switzerland.
(3)European & Developing Countries Clinical Trials Partnership, The Hague,
Netherlands.
(4)Nextco, Oegstgeest, Netherlands.
(5)South African Tuberculosis Vaccine Initiative, Institute of Infectious
Disease and Molecular Medicine, and Division of Immunology, Department of
Pathology, University of Cape Town, Cape Town, South Africa.
To eliminate tuberculosis globally, a new, effective, and affordable vaccine is
urgently needed, particularly for use in adults and adolescents in low-income
and middle-income countries. We have created a roadmap that lists the actions
needed to accelerate tuberculosis vaccine research and development using a
participatory process. The vaccine pipeline needs more diverse immunological
approaches, antigens, and platforms. Clinical development can be accelerated by
validated preclinical models, agreed laboratory correlates of protection,
efficient trial designs, and validated endpoints. Determining the public health
impact of new tuberculosis vaccines requires understanding of a country's demand
for a new tuberculosis vaccine, how to integrate vaccine implementation with
ongoing tuberculosis prevention efforts, cost, and national and global demand to
stimulate vaccine production. Investments in tuberculosis vaccine research and
development need to be increased, with more diversity of funding sources and
coordination between these funders. Open science is important to enhance the
efficiency of tuberculosis vaccine research and development including early and
freely available publication of study findings and effective mechanisms for
sharing datasets and specimens. There is a need for increased engagement of
industry vaccine developers, for increased political commitment for new
tuberculosis vaccines, and to address stigma and vaccine hesitancy. The
unprecedented speed by which COVID-19 vaccines have been developed and
introduced provides important insight for tuberculosis vaccine research and
development.
Copyright © 2022 Elsevier Ltd. All rights reserved.
DOI: 10.1016/S1473-3099(21)00810-0
PMCID: PMC8884775
PMID: 35240041 [Indexed for MEDLINE]
23. Lancet Infect Dis. 2022 Apr;22(4):e121-e127. doi: 10.1016/S1473-3099(21)00613-7. Epub 2022 Feb 25.
Can the GeneXpert MTB/XDR deliver on the promise of expanded, near-patient
tuberculosis drug-susceptibility testing?
Naidoo K(1), Dookie N(2).
Author information:
(1)Centre for the AIDS Programme of Research in South Africa (CAPRISA),
University of KwaZulu-Natal, Durban, South Africa; South African Medical
Research Council (SAMRC) - CAPRISA HIV-TB Pathogenesis and Treatment Research
Unit, Durban, South Africa. Electronic address: kogie.naidoo@caprisa.org.
(2)Centre for the AIDS Programme of Research in South Africa (CAPRISA),
University of KwaZulu-Natal, Durban, South Africa; South African Medical
Research Council (SAMRC) - CAPRISA HIV-TB Pathogenesis and Treatment Research
Unit, Durban, South Africa.
Early diagnosis, including universal drug-susceptibility testing for all
patients with tuberculosis, remains a key priority for tuberculosis elimination
by 2035. The drug-resistant tuberculosis care cascade remains persistently
challenged by substantial gaps in timely diagnosis and treatment of
drug-resistant tuberculosis. Current diagnostics for drug-resistant tuberculosis
are limited with respect to accuracy, time to results, affordability,
suitability for resource-poor endemic settings, and accessibility for use at the
point of care. WHO endorsement of the novel Xpert MTB/XDR assay holds notable
promise for expanding access to testing and rapid diagnosis of tuberculosis drug
resistance. The Xpert MTB/XDR assay detects resistance to isoniazid,
ethionamide, fluoroquinolones, and second-line injectables, and is indicated for
testing in patients with confirmed pulmonary tuberculosis. However, this
iteration of the Xpert MTB/XDR cartridge might have less of an effect than
expected, as WHO has since downgraded the role of second-line injectable agents
in treating drug-resistant tuberculosis, and has revised case definitions of
drug-resistant tuberculosis to incorporate resistance to new drugs. This
Personal View explores the strengths and limitations of the Xpert MTB/XDR assay
in the detection of drug resistance, the assay's ability to inform appropriate
drug-resistant tuberculosis drug selection, and the optimal placement of the
Xpert XDR assay in the laboratory diagnostic workflow.
Copyright © 2022 Elsevier Ltd. All rights reserved.
DOI: 10.1016/S1473-3099(21)00613-7
PMID: 35227392 [Indexed for MEDLINE]
24. J Clin Oncol. 2022 Apr 1;40(10):1127-1129. doi: 10.1200/JCO.22.00051. Epub 2022 Feb 15.
Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I-IIIA
Completely Resected Non-Small-Cell Lung Cancer: ASCO Guideline Rapid
Recommendation Update.
Pisters K(1), Kris MG(2), Gaspar LE(3)(4), Ismaila N(5); Adjuvant Systemic
Therapy and Adjuvant Radiation Therapy for Stage I to IIIA NSCLC Guideline
Expert Panel.
Author information:
(1)MD Anderson Cancer Center, Houston, TX.
(2)Memorial Sloan Kettering Cancer Center, New York, NY.
(3)Banner MD Anderson Cancer Center, Loveland, CO.
(4)University of Colorado School of Medicine, Aurora, CO.
(5)American Society of Clinical Oncology, Alexandria, VA.
ASCO Rapid Recommendations Updates highlight revisions to select ASCO guideline
recommendations as a response to the emergence of new and practice-changing
data. The rapid updates are supported by an evidence review and follow the
guideline development processes outlined in the ASCO Guideline Methodology
Manual. The goal of these articles is to disseminate updated recommendations, in
a timely manner, to better inform health practitioners and the public on the
best available cancer care options.
DOI: 10.1200/JCO.22.00051
PMID: 35167335 [Indexed for MEDLINE]
25. J Clin Oncol. 2022 Apr 20;40(12):1301-1311. doi: 10.1200/JCO.21.01308. Epub 2022 Feb 2.
Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After
Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer.
Spigel DR(1), Faivre-Finn C(2), Gray JE(3), Vicente D(4), Planchard D(5),
Paz-Ares L(6), Vansteenkiste JF(7), Garassino MC(8)(9), Hui R(10), Quantin
X(11), Rimner A(12), Wu YL(13), Özgüroğlu M(14), Lee KH(15), Kato T(16), de Wit
M(17), Kurata T(18), Reck M(19), Cho BC(20), Senan S(21), Naidoo J(22), Mann
H(23), Newton M(24), Thiyagarajah P(23), Antonia SJ(3).
Author information:
(1)Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN.
(2)The University of Manchester and The Christie NHS Foundation Trust,
Manchester, United Kingdom.
(3)H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL.
…
Erratum in
J Clin Oncol. 2022 Jun 10;40(17):1965.
Comment in
J Clin Oncol. 2022 Apr 20;40(12):1271-1274.
PURPOSE: The phase III PACIFIC trial compared durvalumab with placebo in
patients with unresectable, stage III non-small-cell lung cancer and no disease
progression after concurrent chemoradiotherapy. Consolidation durvalumab was
associated with significant improvements in the primary end points of overall
survival (OS; stratified hazard ratio [HR], 0.68; 95% CI, 0.53 to 0.87; P =
.00251) and progression-free survival (PFS [blinded independent central review;
RECIST v1.1]; stratified HR, 0.52; 95% CI, 0.42 to 0.65; P < .0001), with
manageable safety. We report updated, exploratory analyses of survival,
approximately 5 years after the last patient was randomly assigned.
METHODS: Patients with WHO performance status 0 or 1 (any tumor programmed cell
death-ligand 1 status) were randomly assigned (2:1) to durvalumab (10 mg/kg
intravenously; administered once every 2 weeks for 12 months) or placebo,
stratified by age, sex, and smoking history. Time-to-event end point analyses
were performed using stratified log-rank tests. Medians and landmark survival
rates were estimated using the Kaplan-Meier method.
RESULTS: Seven hundred and nine of 713 randomly assigned patients received
durvalumab (473 of 476) or placebo (236 of 237). As of January 11, 2021 (median
follow-up, 34.2 months [all patients]; 61.6 months [censored patients]), updated
OS (stratified HR, 0.72; 95% CI, 0.59 to 0.89; median, 47.5 v 29.1 months) and
PFS (stratified HR, 0.55; 95% CI, 0.45 to 0.68; median, 16.9 v 5.6 months)
remained consistent with the primary analyses. Estimated 5-year rates (95% CI)
for durvalumab and placebo were 42.9% (38.2 to 47.4) versus 33.4% (27.3 to 39.6)
for OS and 33.1% (28.0 to 38.2) versus 19.0% (13.6 to 25.2) for PFS.
CONCLUSION: These updated analyses demonstrate robust and sustained OS and
durable PFS benefit with durvalumab after chemoradiotherapy. An estimated 42.9%
of patients randomly assigned to durvalumab remain alive at 5 years and 33.1% of
patients randomly assigned to durvalumab remain alive and free of disease
progression, establishing a new benchmark for standard of care in this setting.
DOI: 10.1200/JCO.21.01308
PMCID: PMC9015199
PMID: 35108059 [Indexed for MEDLINE]
26. Am J Respir Crit Care Med. 2022 Apr 1;205(7):830-841. doi:
10.1164/rccm.202108-1892OC.
Bacillus Calmette-Guérin Skin Reaction Predicts Enhanced Mycobacteria-Specific
T-Cell Responses in Infants: A Post Hoc Analysis of a Randomized Controlled
Trial.
Pittet LF(1)(2)(3), Fritschi N(4)(5), Tebruegge M(3)(6)(7), Dutta B(8), Donath
S(9)(3), Messina NL(2)(3), Casalaz D(10), Hanekom WA(11), Britton WJ(12),
Robins-Browne R(2)(13), Curtis N(1)(2)(3), Ritz N(3)(4)(5).
Author information:
(1)Infectious Diseases Unit, the Royal Children's Hospital Melbourne, Parkville,
Victoria, Australia.
(2)Infectious Diseases Group and.
(3)Department of Paediatrics and.
…
Comment in
Am J Respir Crit Care Med. 2022 Apr 1;205(7):748-750.
Rationale: Scar formation following bacillus Calmette-Guérin (BCG) vaccination
has been associated with lower all-cause mortality; the relation between scar
and mycobacteria-specific protection against tuberculosis is debated.
Objectives: To evaluate the association between BCG skin reaction and
mycobacteria-specific immune responses. Methods: A post hoc analysis was done
among 214 infants in Australia randomized to vaccination with one of three BCG
vaccine strains (BCG-Denmark, BCG-Japan, or BCG-Russia) given at birth or
BCG-Denmark given at 2 months of age. Measurements and Main Results: BCG skin
reaction size and characteristics 10 weeks after vaccination were related to the
in vitro mycobacteria-specific immune responses measured in stimulated whole
blood. The size and characteristics of the skin reaction correlated positively
with in vitro immune responses, even after adjusting for BCG vaccine strain and
age at vaccination. Specifically, the reaction size and characteristics
correlated with the proportion of mycobacteria-specific polyfunctional CD4+ T
cells after stimulation with BCG and PPD and, to a lesser extent, after
stimulation with Mycobacterium tuberculosis or Mycobacterium ulcerans. A similar
correlation was observed with concentrations of IFN-γ, IL-2, tumor necrosis
factor, and IL-13 in the supernatant after stimulation with BCG, PPD, and M.
tuberculosis and to some degree for the proportions of mycobacteria-specific
polyfunctional CD8+ T cells and CD107+ cytotoxic cells. Conclusions: BCG skin
reaction correlated with the magnitude of mycobacteria-specific T-cell
responses. As T-cell responses play a key role in defense against mycobacteria,
the relationship between BCG scar formation and protection against tuberculosis
should be revisited. This may also extend to the need for BCG revaccination in
scar-negative individuals.Clinical trial registered with
www.australianclinicaltrials.gov.au/clinical-trial-registries
(ACTRN12608000227392).
DOI: 10.1164/rccm.202108-1892OC
PMID: 35007188 [Indexed for MEDLINE]
27. J Clin Oncol. 2022 Apr 20;40(12):1356-1384. doi: 10.1200/JCO.21.02528. Epub 2021 Dec 22.
Management of Stage III Non-Small-Cell Lung Cancer: ASCO Guideline.
Daly ME(1), Singh N(2), Ismaila N(3), Antonoff MB(4), Arenberg DA(5), Bradley
J(6), David E(7), Detterbeck F(8), Früh M(9)(10), Gubens MA(11), Moore AC(12),
Padda SK(13), Patel JD(14), Phillips T(15), Qin A(5), Robinson C(16), Simone CB
2nd(17).
Author information:
(1)University of California, Davis, CA.
(2)Postgraduate Institute of Medical Education & Research, Chandigarh, India.
(3)American Society of Clinical Oncology (ASCO), Alexandria, VA.
…
Comment in
Chirurg. 2022 Apr;93(4):403-404.
PURPOSE: To provide evidence-based recommendations to practicing clinicians on
management of patients with stage III non-small-cell lung cancer (NSCLC).
METHODS: An Expert Panel of medical oncology, thoracic surgery, radiation
oncology, pulmonary oncology, community oncology, research methodology, and
advocacy experts was convened to conduct a literature search, which included
systematic reviews, meta-analyses, and randomized controlled trials published
from 1990 through 2021. Outcomes of interest included survival, disease-free or
recurrence-free survival, and quality of life. Expert Panel members used
available evidence and informal consensus to develop evidence-based guideline
recommendations.
RESULTS: The literature search identified 127 relevant studies to inform the
evidence base for this guideline.
RECOMMENDATIONS: Evidence-based recommendations were developed to address
evaluation and staging workup of patients with suspected stage III NSCLC,
surgical management, neoadjuvant and adjuvant approaches, and management of
patients with unresectable stage III NSCLC.Additional information is available
at www.asco.org/thoracic-cancer-guidelines.
DOI: 10.1200/JCO.21.02528
PMID: 34936470 [Indexed for MEDLINE]
28. J Natl Cancer Inst. 2022 Apr 11;114(4):618-625. doi: 10.1093/jnci/djab224.
The Survival Impact of Second Primary Lung Cancer in Patients With Lung Cancer.
Choi E(1), Luo SJ(1), Aredo JV(2), Backhus LM(3), Wilkens LR(4), Su CC(1), Neal
JW(5)(6), Le Marchand L(4), Cheng I(7), Wakelee HA(5)(6), Han SS(1)(6)(8).
Author information:
(1)Quantitative Sciences Unit, Department of Medicine, Stanford University
School of Medicine, Stanford, CA, USA.
(2)Stanford University School of Medicine, Stanford, CA, USA.
(3)Division of Thoracic Surgery, Department of Cardiothoracic Surgery, Stanford
University School of Medicine, Stanford, CA, USA.
…
BACKGROUND: Lung cancer survivors have a high risk of developing second primary
lung cancer (SPLC), but little is known about the survival impact of SPLC
diagnosis.
METHODS: We analyzed data from 138 969 patients in the Surveillance,
Epidemiology, and End Results (SEER), who were surgically treated for initial
primary lung cancer (IPLC) in 1988-2013. Each patient was followed from the date
of IPLC diagnosis to SPLC diagnosis (for those with SPLC) and last vital status
through 2016. We performed multivariable Cox regression to evaluate the
association between overall survival and SPLC diagnosis as a time-varying
predictor. To investigate potential effect modification, we tested interaction
between SPLC and IPLC stage. Using data from the Multiethnic Cohort Study (MEC)
(n = 1540 IPLC patients with surgery), we evaluated the survival impact of SPLC
by smoking status. All statistical tests were 2-sided.
RESULTS: A total of 12 115 (8.7%) patients developed SPLC in SEER over 700 421
person-years of follow-up. Compared with patients with single primary lung
cancer, those with SPLC had statistically significantly reduced overall survival
(hazard ratio [HR] = 2.12, 95% confidence interval [CI] = 2.06 to 2.17;
P < .001). The effect of SPLC on reduced survival was more pronounced among
patients with early stage IPLC vs advanced-stage IPLC (HR = 2.14, 95% CI = 2.08
to 2.20, vs HR = 1.43, 95% CI = 1.21 to 1.70, respectively;
Pinteraction < .001). Analysis using MEC data showed that the effect of SPLC on
reduced survival was statistically significantly larger among persons who
actively smoked at initial diagnosis vs those who formerly or never smoked
(HR = 2.31, 95% CI = 1.48 to 3.61, vs HR = 1.41, 95% CI = 0.98 to 2.03,
respectively; Pinteraction = .04).
CONCLUSIONS: SPLC diagnosis is statistically significantly associated with
decreased survival in SEER and MEC. Intensive surveillance targeting patients
with early stage IPLC and active smoking at IPLC diagnosis may lead to a larger
survival benefit.
© The Author(s) 2021. Published by Oxford University Press.
DOI: 10.1093/jnci/djab224
PMCID: PMC9002287
PMID: 34893871 [Indexed for MEDLINE]
29. Lancet Infect Dis. 2022 Apr;22(4):507-518. doi: 10.1016/S1473-3099(21)00387-X. Epub 2021 Nov 17.
Tuberculosis screening among ambulatory people living with HIV: a systematic
review and individual participant data meta-analysis.
Dhana A(1), Hamada Y(2), Kengne AP(3), Kerkhoff AD(4), Rangaka MX(5), Kredo
T(6), Baddeley A(7), Miller C(7), Singh S(8), Hanifa Y(9), Grant AD(10),
…
Author information:
(1)Department of Medicine, University of Cape Town, Cape Town, South Africa.
(2)Centre for International Cooperation and Global Tuberculosis Information, The
Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, Tokyo,
Japan; Institute for Global Health, University College London, London, UK.
(3)Non-communicable Diseases Research Unit, South African Medical Research
Council, Cape Town, South Africa.
…
BACKGROUND: The WHO-recommended tuberculosis screening and diagnostic algorithm
in ambulatory people living with HIV is a four-symptom screen (known as the
WHO-recommended four symptom screen [W4SS]) followed by a WHO-recommended
molecular rapid diagnostic test (eg Xpert MTB/RIF [hereafter referred to as
Xpert]) if W4SS is positive. To inform updated WHO guidelines, we aimed to
assess the diagnostic accuracy of alternative screening tests and strategies for
tuberculosis in this population.
METHODS: In this systematic review and individual participant data
meta-analysis, we updated a search of PubMed (MEDLINE), Embase, the Cochrane
Library, and conference abstracts for publications from Jan 1, 2011, to March
12, 2018, done in a previous systematic review to include the period up to Aug
2, 2019. We screened the reference lists of identified pieces and contacted
experts in the field. We included prospective cross-sectional, observational
studies and randomised trials among adult and adolescent (age ≥10 years)
ambulatory people living with HIV, irrespective of signs and symptoms of
tuberculosis. We extracted study-level data using a standardised data extraction
form, and we requested individual participant data from study authors. We aimed
to compare the W4SS with alternative screening tests and strategies and the
WHO-recommended algorithm (ie, W4SS followed by Xpert) with Xpert for all in
terms of diagnostic accuracy (sensitivity and specificity), overall and in key
subgroups (eg, by antiretroviral therapy [ART] status). The reference standard
was culture. This study is registered with PROSPERO, CRD42020155895.
FINDINGS: We identified 25 studies, and obtained data from 22 studies (including
15 666 participants; 4347 [27·7%] of 15 663 participants with data were on ART).
W4SS sensitivity was 82% (95% CI 72-89) and specificity was 42% (29-57).
C-reactive protein (≥10 mg/L) had similar sensitivity to (77% [61-88]), but
higher specificity (74% [61-83]; n=3571) than, W4SS. Cough (lasting ≥2 weeks),
haemoglobin (<10 g/dL), body-mass index (<18·5 kg/m2), and lymphadenopathy had
high specificities (80-90%) but low sensitivities (29-43%). The WHO-recommended
algorithm had a sensitivity of 58% (50-66) and a specificity of 99% (98-100);
Xpert for all had a sensitivity of 68% (57-76) and a specificity of 99% (98-99).
In the one study that assessed both, the sensitivity of sputum Xpert Ultra was
higher than sputum Xpert (73% [62-81] vs 57% [47-67]) and specificities were
similar (98% [96-98] vs 99% [98-100]). Among outpatients on ART (4309 [99·1%] of
4347 people on ART), W4SS sensitivity was 53% (35-71) and specificity was 71%
(51-85). In this population, a parallel strategy (two tests done at the same
time) of W4SS with any chest x-ray abnormality had higher sensitivity (89%
[70-97]) and lower specificity (33% [17-54]; n=2670) than W4SS alone; at a
tuberculosis prevalence of 5%, this strategy would require 379 more rapid
diagnostic tests per 1000 people living with HIV than W4SS but detect 18 more
tuberculosis cases. Among outpatients not on ART (11 160 [71·8%] of 15 541
outpatients), W4SS sensitivity was 85% (76-91) and specificity was 37% (25-51).
C-reactive protein (≥10 mg/L) alone had a similar sensitivity to (83% [79-86]),
but higher specificity (67% [60-73]; n=3187) than, W4SS and a sequential
strategy (both test positive) of W4SS then C-reactive protein (≥5 mg/L) had a
similar sensitivity to (84% [75-90]), but higher specificity than (64% [57-71];
n=3187), W4SS alone; at 10% tuberculosis prevalence, these strategies would
require 272 and 244 fewer rapid diagnostic tests per 1000 people living with HIV
than W4SS but miss two and one more tuberculosis cases, respectively.
INTERPRETATION: C-reactive protein reduces the need for further rapid diagnostic
tests without compromising sensitivity and has been included in the updated WHO
tuberculosis screening guidelines. However, C-reactive protein data were scarce
for outpatients on ART, necessitating future research regarding the utility of
C-reactive protein in this group. Chest x-ray can be useful in outpatients on
ART when combined with W4SS. The WHO-recommended algorithm has suboptimal
sensitivity; Xpert for all offers slight sensitivity gains and would have major
resource implications.
FUNDING: World Health Organization.
© 2022 World Health Organization; licensee Elsevier. This is an Open Access
article published under the CC BY 3·0 IGO license which permits unrestricted
use, distribution, and reproduction in any medium, provided the original work is
properly cited. In any use of this article, there should be no suggestion that
WHO endorses any specific organisation, products or services. The use of the WHO
logo is not permitted. This notice should be preserved along with the article's
original URL.
DOI: 10.1016/S1473-3099(21)00387-X
PMCID: PMC8942858
PMID: 34800394 [Indexed for MEDLINE]
30. Lancet Infect Dis. 2022 Apr;22(4):496-506. doi: 10.1016/S1473-3099(21)00470-9. Epub 2021 Nov 12.
Assessment of epidemiological and genetic characteristics and clinical outcomes
of resistance to bedaquiline in patients treated for rifampicin-resistant
tuberculosis: a cross-sectional and longitudinal study.
Ismail NA(1), Omar SV(2), Moultrie H(3), Bhyat Z(3), Conradie F(4), Enwerem
M(5), Ferreira H(6), Hughes J(7), Joseph L(3), Kock Y(8), Letsaolo V(3),
Maartens G(9), Meintjes G(9), Ngcamu D(3), Okozi N(3), Padanilam X(10), Reuter
A(11), Romero R(12), Schaaf S(7), Te Riele J(13), Variava E(14), van der Meulen
M(3), Ismail F(15), Ndjeka N(8).
Author information:
(1)Centre for Tuberculosis, National Institute for Communicable Diseases,
Johannesburg, South Africa; Department of Medical Microbiology, University of
Pretoria, Pretoria, South Africa; Faculty of Health Sciences, University of
Witwatersrand, Johannesburg, South Africa.
(2)Centre for Tuberculosis, National Institute for Communicable Diseases,
Johannesburg, South Africa. Electronic address: shaheedvo@nicd.ac.za.
(3)Centre for Tuberculosis, National Institute for Communicable Diseases,
Johannesburg, South Africa.
…
Comment in
Lancet Infect Dis. 2022 Feb;22(2):166-167.
Lancet Infect Dis. 2022 Feb;22(2):166.
Comment on
Lancet Infect Dis. 2022 Apr;22(4):432-433.
BACKGROUND: Bedaquiline improves outcomes of patients with rifampicin-resistant
and multidrug-resistant (MDR) tuberculosis; however, emerging resistance
threatens this success. We did a cross-sectional and longitudinal analysis
evaluating the epidemiology, genetic basis, and treatment outcomes associated
with bedaquiline resistance, using data from South Africa (2015-19).
METHODS: Patients with drug-resistant tuberculosis starting bedaquiline-based
treatment had surveillance samples submitted at baseline, month 2, and month 6,
along with demographic information. Culture-positive baseline and post-baseline
isolates had phenotypic resistance determined. Eligible patients were aged 12
years or older with a positive culture sample at baseline or, if the sample was
invalid or negative, a sample within 30 days of the baseline sample submitted
for bedaquiline drug susceptibility testing. For the longitudinal study, the
first surveillance sample had to be phenotypically susceptible to bedaquiline
for inclusion. Whole-genome sequencing was done on bedaquiline-resistant
isolates and a subset of bedaquiline-susceptible isolates. The National
Institute for Communicable Diseases tuberculosis reference laboratory, and
national tuberculosis surveillance databases were matched to the Electronic
Drug-Resistant Tuberculosis Register. We assessed baseline resistance
prevalence, mutations, transmission, cumulative resistance incidence, and odds
ratios (ORs) associating risk factors for resistance with patient outcomes.
FINDINGS: Between Jan 1, 2015, and July 31, 2019, 8041 patients had surveillance
samples submitted, of whom 2023 were included in the cross-sectional analysis
and 695 in the longitudinal analysis. Baseline bedaquiline resistance prevalence
was 3·8% (76 of 2023 patients; 95% CI 2·9-4·6), and it was associated with
previous exposure to bedaquiline or clofazimine (OR 7·1, 95% CI 2·3-21·9) and
with rifampicin-resistant or MDR tuberculosis with additional resistance to
either fluoroquinolones or injectable drugs (pre-extensively-drug resistant
[XDR] tuberculosis: 4·2, 1·7-10·5) or to both (XDR tuberculosis: 4·8, 2·0-11·7). Rv0678 mutations were the sole genetic basis of phenotypic resistance. Baseline resistance could be attributed to previous bedaquiline or clofazimine exposure in four (5·3%) of 76 patients and to primary transmission in six (7·9%). Odds of successful treatment outcomes were lower in patients with baseline bedaquiline resistance (0·5, 0·3-1). Resistance during treatment developed in 16 (2·3%) of 695 patients, at a median of 90 days (IQR 62-195), with 12 of these 16 having pre-XDR or XDR.
INTERPRETATION: Bedaquiline resistance was associated with poorer treatment
outcomes. Rapid assessment of bedaquiline resistance, especially when patients
were previously exposed to bedaquiline or clofazimine, should be prioritised at
baseline or if patients remain culture-positive after 2 months of treatment.
Preventing resistance by use of novel combination therapies, current treatment
optimisation, and patient support is essential.
FUNDING: National Institute for Communicable Diseases of South Africa.
Copyright © 2022 Elsevier Ltd. All rights reserved.
DOI: 10.1016/S1473-3099(21)00470-9
PMID: 34780706 [Indexed for MEDLINE]






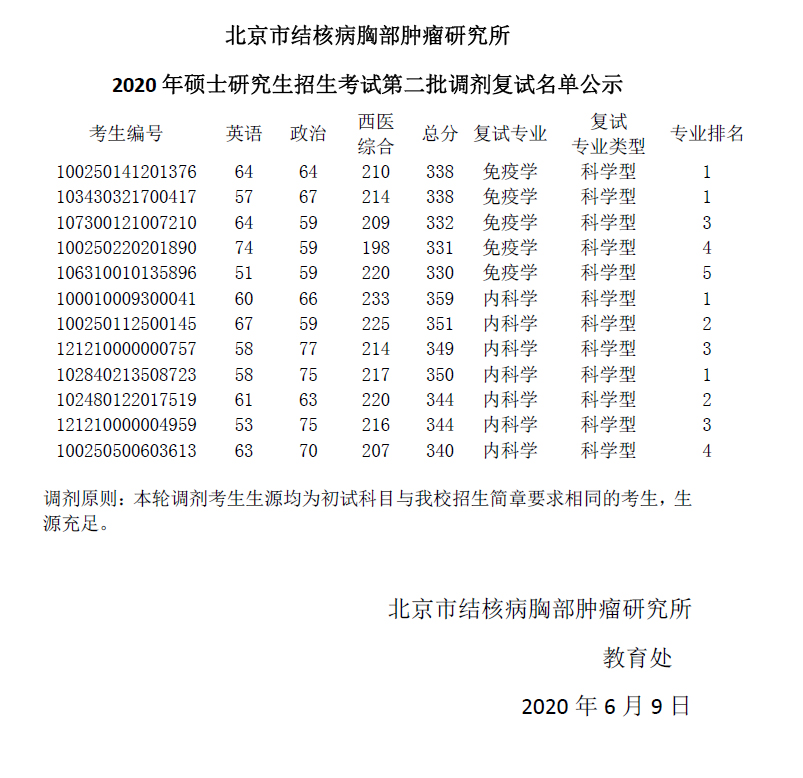


.jpg)











